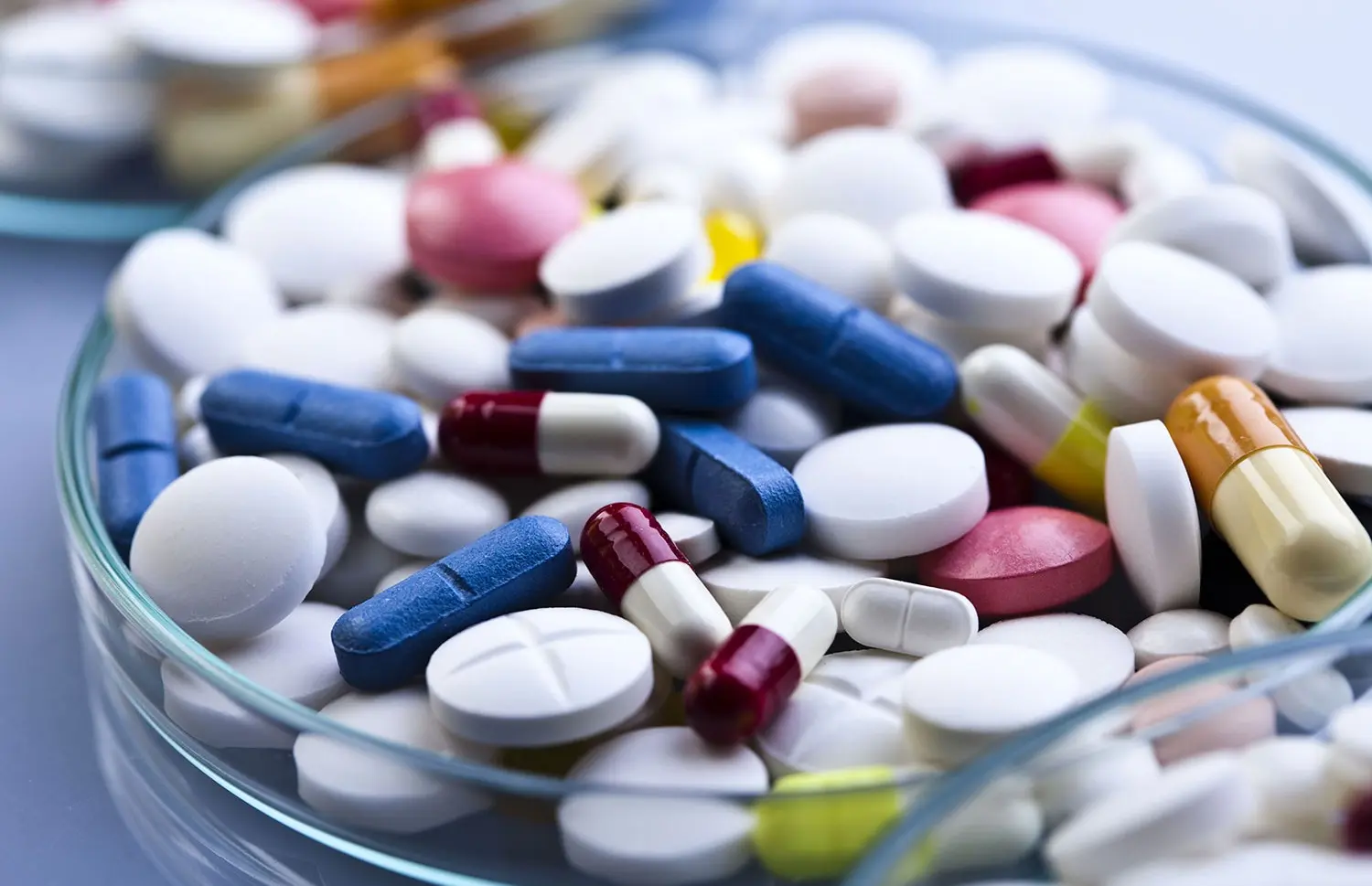
Registration, Promotion and Distribution

One of the most attractive pharmaceutical markets - the EAEU market - is more accessible than you expect. We are always ready to help you in solving a set of tasks or individual issues in this area.
Work Stages

Production Site Certification
Preparation of the production site for the EAEU GMP Rules inspection, including preliminary audit and submission of the necessary documents to the relevant state authorities
Creation of all necessary documentation related to production processes and quality system policy
GMP inspection support
Creation of all necessary documentation related to production processes and quality system policy
GMP inspection support

Evidence Base and Registration
Organization of preclinical and clinical studies in accredited laboratories and testing centers
Registration dossier preparation
Support of the registration procedure
Registration dossier preparation
Support of the registration procedure

Distribution and promotion support
Registration of trade representative offices and organizations
Search and organization of negotiations with distributors
Organization of marketing and advertising events
Search and organization of negotiations with distributors
Organization of marketing and advertising events
Contact Us
Phone/Whatsapp/Telegram
+7 996 716 83 66
E-mail
info@mpmadviser.com